Chapter 3: Fluorescence & Fluorochromes
You are here
3.1 Introduction
When a compound absorbs light (and hence energy) electrons are raised from the ground state to an excited state. Electrons return to the ground state by a variety of transitions which may involve the emission of a quantum of light (radiative transition). This effect is termed fluorescence (Figure 3.1). The emitted light will always be of lower energy, and hence longer wavelength, than the exciting light. The energy may also be lost by nonradiative processes and eventually dissipated as heat.
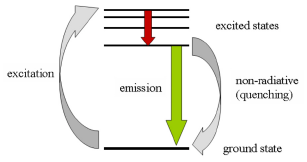
Figure 3.1. The absorption and emission of light during fluorescence. After excitation by light absorption, the electron moves to an excited state of lower energy (red arrow). When it drops to the ground state (green arrow) light is emitted.
Because the colour of the exciting and emitting light are different, they can be separated from one another by using optical filters.
Fluorescence detection is a sensitive technique because a positive signal is observed against a negative background. The detection of up to 14 compounds fluorescing at different wavelengths permits multiparametric analysis of cells and has greatly increased the power of flow cytometry. The majority of applications use up to four fluorescences.
3.2 Properties of a fluorochrome
The important properties of a fluorochrome are its absorption spectrum, its extinction coefficient at a wavelength convenient for excitation, its emission spectrum and its quantum efficiency. The latter is the number of photons emitted for every photon absorbed. The difference between the maxima in the wavelengths of absorption and emission is known as the Stoke’s shift. The higher the Stoke’s shift, the greater the separation between the exciting and the emitted light.
The properties of a fluorochrome will depend on its environment. For example, propidium iodide (PI), which is used to stain DNA, is only weakly fluorescent in water; on intercalating with DNA, the fluorescence increases 50 fold due to the hydrophobic environment. Some fluorochromes, such as fluorescein, are sensitive to pH. There are other compounds whose fluorescent spectrum changes according to the concentration of certain ions, such as Ca++ (see Section 3.3.3).
Fluorescence can also be quenched by an interaction with another molecule in which the energy is dissipated by a non-radiative transition. This can be illustrated by the bis-benzimadazole dye, Hoechst 33342, which binds to DNA giving blue fluorescence on excitation with UV. However, if DNA is labelled with 5'-bromodeoxyuridine, the fluorescence of the dye is quenched by the bromine atom. If a compound is over-labelled with a fluorochrome, fluorescence can also be quenched by interactions between the molecules of fluorochrome. This effect can be observed when liposomes are loaded with fluorescein; fluorescence decreases with increasing dye concentration above about 20 µM.
If two fluorochromes are closely associated, energy transfer can occur whereby excitation of one compound causes the other to fluoresce. For example, if the rhodamine derivative, Texas Red, is bound to the fluorescent protein, phycoerythrin (PE), excitation of PE will lead to emission of light from Texas Red. The effective emission maximum will have been shifted from that of PE (576 nm) to that of Texas Red (620 nm). For energy transfer to occur, the acceptor molecule has to have an absorption spectrum that overlaps with the emission spectrum of the donor molecule (Figure 3.2).
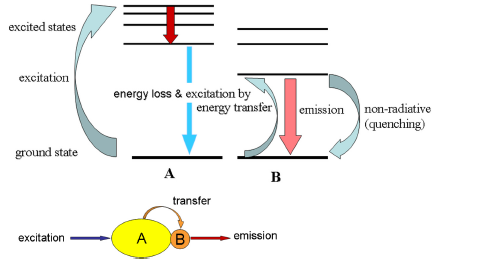
Figure 3.2. Energy transfer. Molecule A absorbs light and transfers the energy to molecule B, which fluoresces.
3.3 Fluorochromes used in flow cytometry
In general, there are two classes of fluorochrome used in flow cytometry - those which bind non-covalently to structures within the cell and those which are covalently bound to other probes. The fluorescent proteins, such as Green Fluorescent Protein, (GFP) form a special category. They are used as reporter molecules when introducing a gene construct into a cell.
3.3.1 Compounds used to label proteins covalently.
The probe used is commonly an antibody but other proteins, such as a lectin, hormone, avidin or streptavidin, or even c-DNA may be labelled. The largest application of labelled proteins is for immunofluorescence (see Chapter 5).
Some of the more widely used fluorescent labels are listed in Table 3.1. A selection of these labels could be used in the arrangement shown in Figure 2.7.
Table 3.1. Some fluorochromes used to label proteins
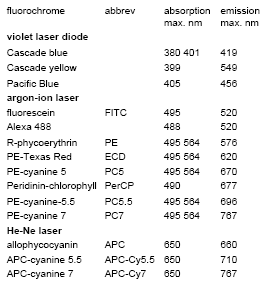
The most common fluorochrome used to label proteins is fluorescein isothiocyanate, the isothiocyanate group reacts with the amino groups on the lysine residues in the protein. The number of labelled proteins that can be used has been greatly extended by the use of tandem dyes. Examples include conjugates of PE and allophycocyanin (APC) with various cyanine dyes (Table 3.1).
A wide range of directly labelled monoclonal antibodies and labelled anti-Igs are available from several manufacturers. It is seldom necessary to label proteins in the laboratory and the reader is recommended to purchase labelled reagents.
3.3.2 Quantum dots
Quantum dots are fluorescent nanocrystals; those used in flow cytometry are 10 - 20 nm in size. They have a wide absorption spectrum and so can be excited by a range of different wavelengths. Their emission wavelength depends on their size and can be from blue through to deep red. Their emission spectra are very narrow compared to the emission spectra of conventional dyes, reducing the overlap between the different fluorescences. (The significance of this is discussed in Chapter 5). Their use is particularly attractive in immunology enabling a large number of T cells subsets to be enumerated in a single experiment (Chattopadhyay, 2006). At present, their use is limited but is certain to increase as the technology for linking them to different probes improves and the price reduces.
3.3.3 Probes for nucleic acids
Table 3.2. Some fluorochromes used to stain nucleic acids fluorochrome abbreviation absorption emission.
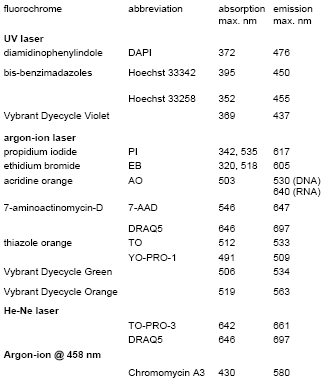
The fluorochromes described in this section bind stoichiometrically to nucleic acids so that they can be used for quantitative measurement. This is essential for the measurement of ploidy and the cell cycle (see Chapter 6).
The anthraquinone, DRAQ5, and bis-benzimadazoles, such as Hoechst 33342, cross an intact plasma membrane and can be used to visualize the cell cycle in viable cells. With the other compounds listed, cells have to be fixed or permeabilised before staining for DNA .
Propidium iodide (PI) is the dye most widely used as a DNA stain. PI (and the closely related compound, ethidium bromide) intercalates between the bases in double stranded nucleic acids. It is excited by blue light giving red fluorescence. With an argon-ion laser tuned to 488 nm, PI can be used in combination with fluorescein, which makes it particularly useful for simultaneous measurement of antibody binding and DNA content. PI also binds to double stranded RNA, which has to be removed by treatment with RNase.
DRAQ5 has an absorption maximum at 646 nm. However, it has a broad absorption spectrum and a high extinction coefficient and it absorbs sufficient light at 488 nm to permit its use with an argon-ion laser.
The bis-benzimadazoles (usually referred to by the number given to them by the original manufacturer, Hoechst) bind preferentially to AT-rich regions in the small groove of double-stranded DNA and fluoresce blue when excited by UV light. The antibiotic, chromomycin A3, binds to the GC-rich regions of DNA. It has been used in conjunction with Hoechst 33258 to resolve chromosomes of similar size but with different AT/GC ratios (see Chapter 9, Section 9.12).
4',6-diamidino-2-phenylindole (DAPI) has been used extensively to label DNA for excitation with UV; it is often the stain of choice in instruments employing a mercury arc lamp.
A new series of cell-permeant dyes has recently been introduced by Invitrogen with the proprietary name, Vybrant DyeCycle (VD). VD Violet can be excited either with UV or with a solid state violet laser at 410 nm. VD Orange can be excited at 488 nm or at 530 nm.
3.3.4 Fluorescent proteins
Green fluorescent protein (GFP) was originally isolated from the jellyfish, Aequorea victoria. It is excited at 488 nm and fluoresces green. The GFP gene is used as a reporter for gene transfection. There are now a variety of mutants available, emitting at different colours. One of the major uses for these proteins is for the purification of transfected cells using the cell sorter.
3.3.5 Other reporter molecules
Some applications using other reporter molecules are outlined in Chapter 10. A few general principles are described below.
Loading the cell. The internal milieu of the cell can be explored using compounds whose fluorescence is sensitive to the environment. Such reporter molecules must be introduced into the cell and, once there, they should not migrate out. This is not a problem if the probe binds tightly to a cell component (e.g. Hoechst 33342 to DNA) or if the partitioning of the probe between the cytoplasm and the medium is being measured (e.g. probes of plasma membrane potential, see Chapter 9, Section 9.3.4). With other probes a different strategy must be found.
Many reporter molecules are charged and, as such, have difficulty in crossing the plasma membrane; a general procedure adopted for loading cells is to mask the charge by preparing an ester. The uncharged molecule will diffuse into the cell in which non-specific esterases convert the reporter molecule to its original non-permeant form (Figure 3.3).
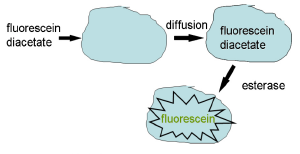
Figure 3.3. Method for loading charged dyes into cells.
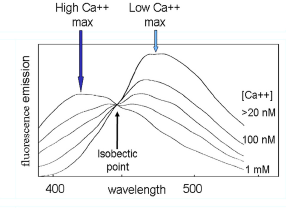
Figure 3.4. The change in emission spectrum of indo-1 with change in calcium concentration; excitation wavelength: 360 nm. Redrawn with permission from Rabinovitch and June (2000).
Ratio measurements. Once in the cell, the amount of a reporter molecule present, and hence its total fluorescence, will generally depend on the size of the cell. Changes in the amount of light emitted on a single cell basis cannot be measured; only a general shift in the population can be recorded. It is therefore useful to use a probe that shows a shift in the wavelength of emission. Fluorescence is measured at two wavelengths and the ratio calculated for each cell. This ratio will be independent of the total amount of fluorochrome in the cell.
For example, the calcium indicator, indo-1, has an emission maximum of about 490 nm in the absence of Ca++; in the presence of 1 mM Ca++, this shifts down to about 410 nm (Figure 3.4). It can be loaded into cells as its acetoxymethyl ester. Indo-1 is excited with UV and changes in the concentration of intracellular Ca++ recorded by observing fluorescence either side of the isobectic point (for example, at 400 and 520 nm).
Reporter molecules showing a shift in emission spectrum have also been used to measure intracellular magnesium ions and pH (see Section 10.4).
References
Chattopadhyay, P.K. et al. (2006) Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nat. Med. 12, 972-977.
Rabinovitch, P.S. and June, C.H. (2000) Intracellular ionised calcium, magnesium, membrane potential and, pH. In: Flow Cytometry. A Practical Approach. 3rd edition, (ed. Ormerod, M.G.), pp. 203-233. Oxford University Press, Oxford.
The online catalogue from Invitrogen is good source of information about most fluorescent probes used in flow cytometry.
For information about DRAQ5, see the Biostatus Web site (www.biostatus.com).
